Emma’s research is focussed on developing a better understanding of the solid state. She is using a three pronged attack, studying the crystallisation of chalcones; examining the effect of temperature on a material that undergoes a phase transition, and investigating the World’s Favourite Space Group, P21/c. She is using a wide range of probes, including the Cambridge Structural Database, dSNAP, Laue Diffraction, Solid State NMR and Variable Temperature Single Crystal Diffraction as well as collecting data at Diamond. When not fighting to grow crystals or preparing dinner for the team at Diamond, she enjoys dancing, but refuses to perform for the group.
Emma’s research is focussed on developing a better understanding of the solid state. She is using a three pronged attack, studying the crystallisation of chalcones; examining the effect of temperature on a material that undergoes a phase transition, and investigating the World’s Favourite Space Group, P21/c. She is using a wide range of probes, including the Cambridge Structural Database, dSNAP, Laue Diffraction, Solid State NMR and Variable Temperature Single Crystal Diffraction as well as collecting data at Diamond. When not fighting to grow crystals or preparing dinner for the team at Diamond, she enjoys dancing, but refuses to perform for the group.
 Jeremy’s research revolves around using various techniques (NMR, crystallography and DFT calculations) to provide information about exactly where hydrogen atoms are located in crystal structures, and exploring the merits and shortcomings of the various techniques for this purpose. When he isn’t trying to persuade a supercomputer to calculate what he wants, he enjoys playing the trumpet with the Oxford University Big Band, plays squash and designs prize-winning posters.
Jeremy’s research revolves around using various techniques (NMR, crystallography and DFT calculations) to provide information about exactly where hydrogen atoms are located in crystal structures, and exploring the merits and shortcomings of the various techniques for this purpose. When he isn’t trying to persuade a supercomputer to calculate what he wants, he enjoys playing the trumpet with the Oxford University Big Band, plays squash and designs prize-winning posters.
 As part of Age Concern, an EPSRC-funded exploration into the world of modern crystallography, Dave is developing a fast, live, configurable chemical structure matching algorithm to identify discrepancies between an expected structure and a real crystallographic structure. Implemented in his software “matchbOx”, Dave is augmenting the software capabilities to automatically handle certain cases of crystal disorder, whilst enjoying the best music the 80s has to offer. When not advancing the boundaries of science, Dave enjoys weight-training, making music and working on pet programming projects.
As part of Age Concern, an EPSRC-funded exploration into the world of modern crystallography, Dave is developing a fast, live, configurable chemical structure matching algorithm to identify discrepancies between an expected structure and a real crystallographic structure. Implemented in his software “matchbOx”, Dave is augmenting the software capabilities to automatically handle certain cases of crystal disorder, whilst enjoying the best music the 80s has to offer. When not advancing the boundaries of science, Dave enjoys weight-training, making music and working on pet programming projects.
Amber is the Chemical Crystallography Service Manager. See her Departmental web-page for further information.
 David was the Head of Chemical Crystallography prior to his retirement in 2010. See his Departmental web-page for further information.
David was the Head of Chemical Crystallography prior to his retirement in 2010. See his Departmental web-page for further information.
 Richard is the Head of the Chemical Crystallography.
Richard is the Head of the Chemical Crystallography.
Telephone: +44/(0) 1865 275963.
CrystEngComm (2011), 13(8) 2923-2929. [ doi:10.1039/c0ce00709a ]
A series of racemic or stereochemically labile chiral borate anions based on the 2,20-biphenol motif was
investigated. All borates were homochiral in the solid state, although in some cases the heterochiral
diastereomers were computed to be thermodynamically preferred (DFT). The crystallographic
preference for the homochiral diastereomer was attributed to its lower bulk, higher molecular
symmetry, and the therewith associated better packing ability.
Presented by: Dr. Andrew D. Schwarz & Liban M. A. Saleh
Research Leader: Prof. Philip Mountford & Prof. Simon Aldridge
Published: Journal of the American Chemical Society
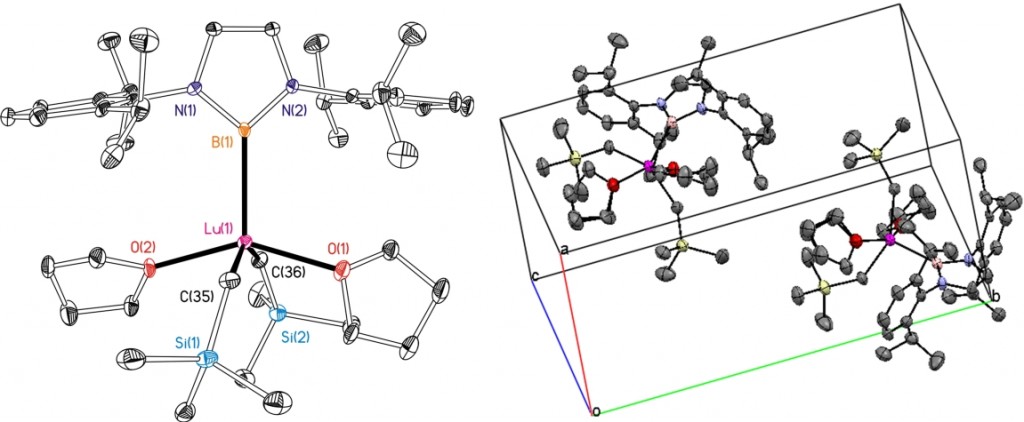
Transition-metal boryl compounds (L)M(BX2)x, containing 2‑center, 2‑electron σ‑bonds have been a topic of outstanding interest due to pivotal roles in a variety of catalytic and stoichiometric transformations, e.g. hydroboration and diboration of C–C π-bonds, and functionalization of alkane and arene C‑H bonds. To date, virtually all boryl complexes have been prepared either by B‑X (X = H or halogen) or B‑B oxidative addition to a low oxidation state (L)M species, or by nucleophilic attack of a [(L)M]‑ anion on a XBR2 or related source of the boryl moiety. However, utilisation of the nucleophilic Li{B(NArCH)2}(THF)2 (Ar = 2,6‑C6H3iPr2), allows access to rare earth metal boryl compounds. Data were collected on a small colourless crystal (0.05 × 0.05 × 0.05 mm) using the new Oxford Diffraction (Agilent) SuperNova diffractometers and copper radiation.
Presented by: Nicholas H. Evans & Christopher J. Serpell
Research Leader: Prof. Paul D. Beer
Published: Angewandte Chemie International Edition
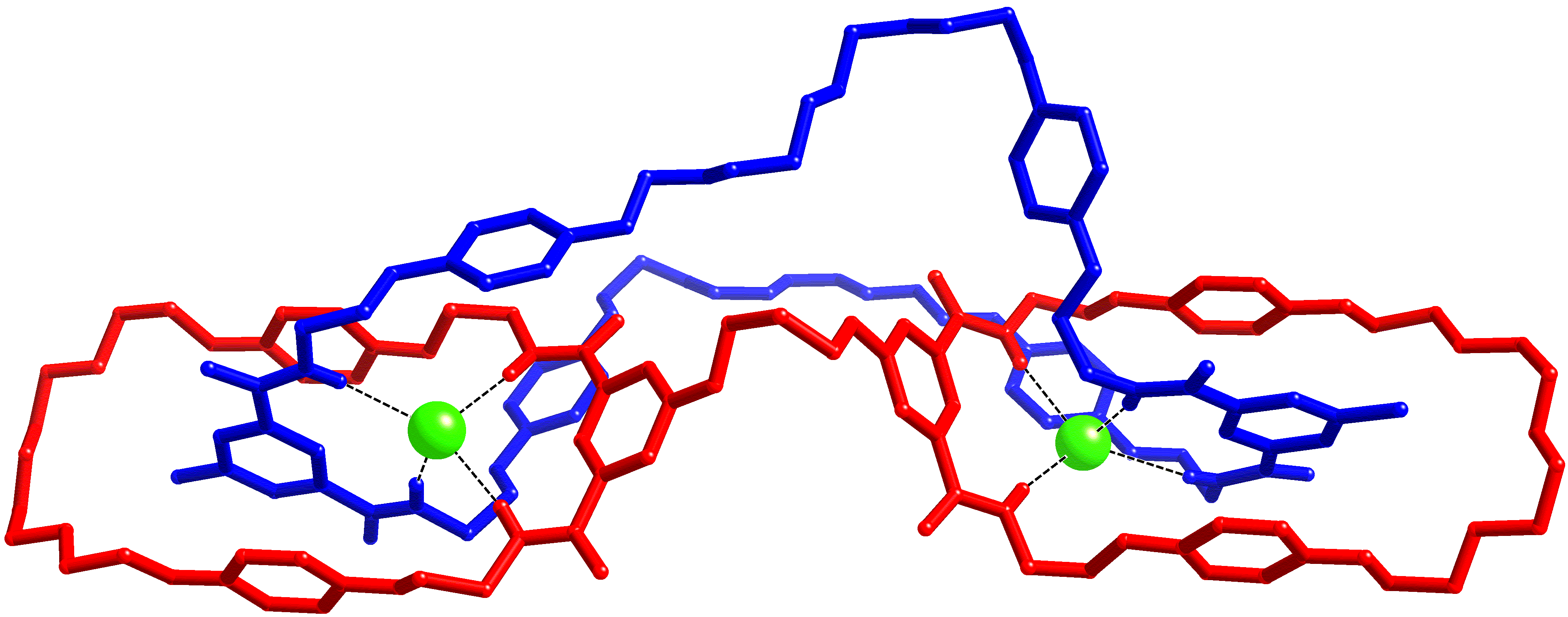
Catenanes and rotaxanes are highly attractive targets for the supramolecular chemist due to their potential uses as molecular machines or as selective hosts for ionic and molecular guests. This molecule was synthesised via chloride anion templation and crystals grown by slow diffusion of diisopropyl ether into a chloroform/catenane solution. Data were collected on I19 at Diamond. This first handcuff catenane structure provided proof of the topology, also revealing potential further uses: the degree of slack in the large macrocycle could allow controlled rotation within the handcuff, and the large number of oxygen atoms in the cavity formed by the handcuff linker and large macrocycle could be used to bind cations.