Chirality (2008), 20(7), 863-870. [ doi:10.1002/chir.20561 ]
3 alpha,12 alpha-Dihydroxy-5 beta-cholan-24-oic acid (deoxycholic acid DCA) is able to discriminate between the R- and S-enantiomers of camphorquinone and endo-(+)-3-bromocamphor and select only the S-enantiomers from a racemic mixture. DCA forms novel well ordered 1:l adducts with (1S)-(+)-camphorquinone and (1S)-endo-(-)-3-bromocamphor, both of which have been characterized by single crystal X-ray diffraction (SXRD). When DCA is cocrystallized with (RS)-camphorquinone and (RS)-endo-3-bromocamphor, 1:1 adducts of the S-enantiomers are produced together with crystals of the free racemic guest. In contrast, in the absence of (1S)-(+)-camphorquinone, DCA forms a 2:1 adduct with (1R)-(-)-camphorquinone. In this 2:1 adduct the guest is disordered at ambient temperature and undergoes a phase change in the region 160-130 K similar to that observed for the ferrocene adduct, but with only partial ordering of the guest. The SXRD structure of the low temperature form and the variable temperature C-13 CP/MAS NMR are reported. Cocrystallizing DCA with (1R)-endo-(+)-3-bromocamphor gives the free guest and a glassy solid.
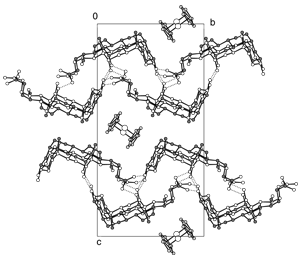
Packing diagram of (deoxycholic acid)2:ferrocene at 100K viewed along the crystallographic a-axis; hydrogen bonds are represented by dotted lines
