Z’ > 1
Z’ > 1:Â Just a Nuisance, or Something More Interesting?
For many discrete-molecule materials, the molecules pack together to form crystals in a relatively simple way determined by the Space Group. Such materials are said to have Z’=1 (Z-prime). Increasingly, crystallographers are beginning to find crystals in which the basic building block is not just one molecule, but several molecules taken together. Such materials are said to have Z’>1.
These materials must be studied for two reasons. The most urgent is that they can pose serious problems for the crystallographer, so that new computational tools need to be developed. More fundamental is the question of why these molecules choose to crystallise in such complicated patterns.
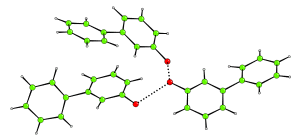
3-hydroxybiphenyl is a simple molecule with a complex crystal structure. It turns out that in the crystal, the molecules hang about in groups of three, with the three molecules hydrogen bonded to each other. The hydrogen bonded network cannot extend any further. A fourth molecule cannot get its hydroxyl group sufficiently close to those in the trimer because of steric restrictions.