Presented by: Nicholas G. White & Dr. Fabiola Zapata
Research Leader: Prof. Paul D. Beer
Published: Journal of the American Chemical Society
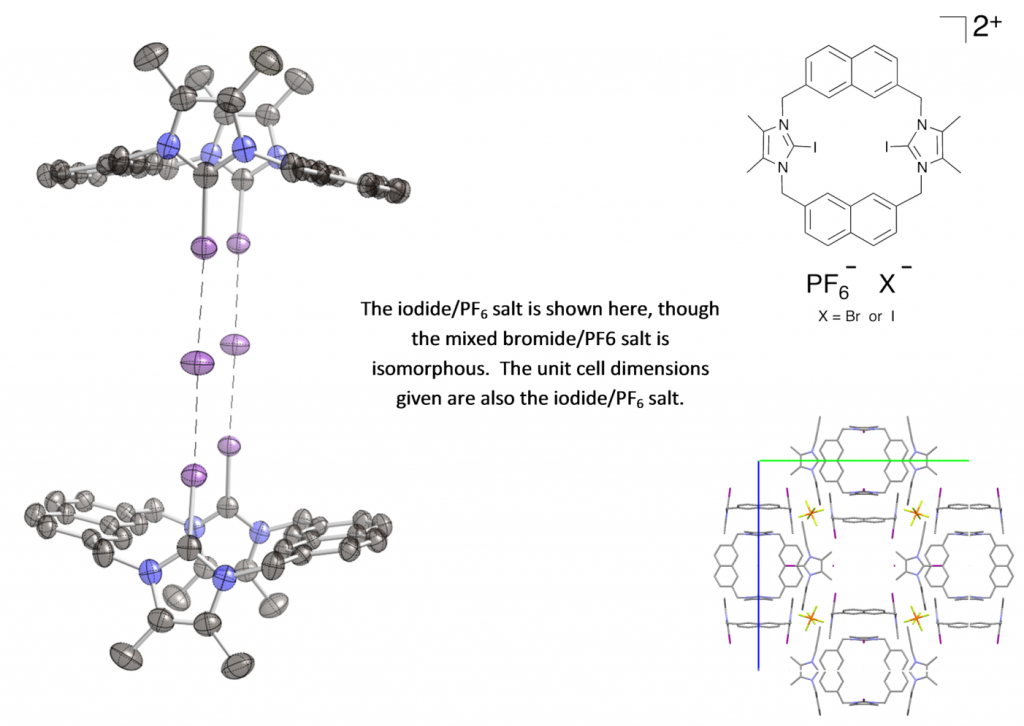
Both the mixed bromide/PF6 and mixed iodide/PF6 salt of this bis-iodoimidazolium macrocycle crystallize in the unusual cubic spacegroup I m -3 (there are currently only six organic structures in this spacegroup in the CSD). The macrocycle binds anions strongly and selectively in competitive methanol/water solvent mixtures, with anions being bound solely by halogen-bonding interactions. In the solid state, the both the bromide and iodide salts of the macrocycle exist as a dimer, with two macrocycles each pointing at two halide anions. Experiments are being undertaken to see if this arrangement occurs in solution.