Will is working with Nick Rees, looking into the host/guest complexes of deoxycholic acid. Using variable temperature single crystal diffraction, along with Solid State NMR and Differential Scanning Calorimetry the project is particularly focused on phase changes within the crystal as a function of temperature. When not in the lab he enjoys long lunches, and competing for the Oxford Modern Pentathlon team.
Will is working with Nick Rees, looking into the host/guest complexes of deoxycholic acid. Using variable temperature single crystal diffraction, along with Solid State NMR and Differential Scanning Calorimetry the project is particularly focused on phase changes within the crystal as a function of temperature. When not in the lab he enjoys long lunches, and competing for the Oxford Modern Pentathlon team.
 The triennial Congress of the International Union of Crystallography was held in Madrid, Spain at the end of August 2011. Chem. Cryst. was well represented with several talks and posters including:
The triennial Congress of the International Union of Crystallography was held in Madrid, Spain at the end of August 2011. Chem. Cryst. was well represented with several talks and posters including:
Richard I. Cooper
Optimising X-ray Experiment Strategy on-the-fly based on Feedback from Automated Structure Solution (Presentation)
Amber L. Thompson
Battle of the Titans:Â Atlas vs. Eos and Other Stories (Invited presentation at the Agilent Technologies User Forum)
Jevgenij A. Raskatov, John M. Brown & Amber L. Thompson
Chiral Selection in the Formation of Borates from Racemic Binaphthols and related Diols (Poster)
Amber L. Thompson
Influencing Absolute Structure Determination (Poster)
CrystEngComm (2011), 13(8) 2923-2929. Â Â [ doi:10.1039/c0ce00709a ]
A series of racemic or stereochemically labile chiral borate anions based on the 2,20-biphenol motif was
investigated. All borates were homochiral in the solid state, although in some cases the heterochiral
diastereomers were computed to be thermodynamically preferred (DFT). The crystallographic
preference for the homochiral diastereomer was attributed to its lower bulk, higher molecular
symmetry, and the therewith associated better packing ability.
Chirality (2008), 20(7), 863-870. Â Â [ doi:10.1002/chir.20561 ]
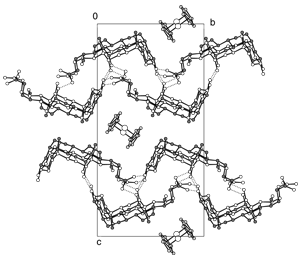
3 alpha,12 alpha-Dihydroxy-5 beta-cholan-24-oic acid (deoxycholic acid DCA) is able to discriminate between the R- and S-enantiomers of camphorquinone and endo-(+)-3-bromocamphor and select only the S-enantiomers from a racemic mixture. DCA forms novel well ordered 1:l adducts with (1S)-(+)-camphorquinone and (1S)-endo-(-)-3-bromocamphor, both of which have been characterized by single crystal X-ray diffraction (SXRD). When DCA is cocrystallized with (RS)-camphorquinone and (RS)-endo-3-bromocamphor, 1:1 adducts of the S-enantiomers are produced together with crystals of the free racemic guest. In contrast, in the absence of (1S)-(+)-camphorquinone, DCA forms a 2:1 adduct with (1R)-(-)-camphorquinone. In this 2:1 adduct the guest is disordered at ambient temperature and undergoes a phase change in the region 160-130 K similar to that observed for the ferrocene adduct, but with only partial ordering of the guest. The SXRD structure of the low temperature form and the variable temperature C-13 CP/MAS NMR are reported. Cocrystallizing DCA with (1R)-endo-(+)-3-bromocamphor gives the free guest and a glassy solid.
Ibby completed a D.Phil. in Chem. Cryst. in 2002 working on clathrates of deoxycholic acid. He now works in Malaysia where he is the proud owner of a new diffractometer and visits Oxford when he can.
 Nick is Inorganic Nuclear Magnetic Resonance Manager. Although he comes from The Dark Side, solid state NMR has its uses and he not only works closely with us, but he is also continuing the work he carried out with Keith Prout on deoxycholic acid complexes. As such he is an honorary member of Chem. Cryst.
Nick is Inorganic Nuclear Magnetic Resonance Manager. Although he comes from The Dark Side, solid state NMR has its uses and he not only works closely with us, but he is also continuing the work he carried out with Keith Prout on deoxycholic acid complexes. As such he is an honorary member of Chem. Cryst.