Presented by:Â Nicholas H. Evans & Christopher J. Serpell
Research Leader:Â Prof. Paul D. Beer
Published: Angewandte Chemie International Edition
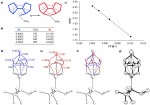
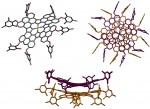
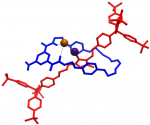
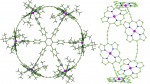
Catenanes and rotaxanes are highly attractive targets for the supramolecular chemist due to their potential uses as molecular machines or as selective hosts for ionic and molecular guests. This molecule was synthesised via chloride anion … Read the rest